DOI: 10.12809/hkmj144390
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Babesiosis acquired from a pet dog: a second reported case in Hong Kong
Jacky MC Chan, FHKCP, FHKAM (Medicine); KY Tsang, FHKAM (Medicine), FRCP (Glasg & Edin);
Thomas SH Chik, MB, ChB, MRCP(UK); WS Leung, FHKCP, FHKAM (Medicine); Owen TY Tsang, FHKAM (Medicine), FRCP (Edin)
Department of Medicine and Geriatrics, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr Jacky MC Chan (cmc061@ha.org.hk)
A 61-year-old Chinese man was admitted to our
hospital in June 2012 with a 2-week history of fever
and chills. After admission, he developed hypotension
and respiratory distress and was transferred to the
intensive care unit (ICU) for further management.
He frequently commuted between New York,
Hong Kong, and Shanghai for business. He lived in
his own house in New York and had a dog. Three
weeks before onset of his symptoms, he swept his
basement where his dog spent most of its time. He
was not aware of any tick bite.
He was a chronic smoker but enjoyed good past
health. His symptoms began with fever and chills
while in China. He also experienced malaise and a
mild dry cough. He had no skin rash, or abdominal or
urinary symptoms. A diagnosis of upper respiratory
tract infection was made and he was given a course
of piperacillin-tazobactam during hospitalisation
in China. Symptoms persisted, however, and he
presented to a public hospital in Hong Kong. Initial
blood tests revealed a normochromic normocytic
anaemia (haemoglobin, 81 g/L) with reticulocytosis,
marked thrombocytopenia, and moderate renal
impairment. Blood smear revealed the presence of
intra-erythrocytic ring-form parasites, suggestive
of Plasmodium falciparum infection (Fig 1). Chest
X-ray showed bilateral extensive abnormal lung
infiltrates. He was transferred to our hospital for
further management.
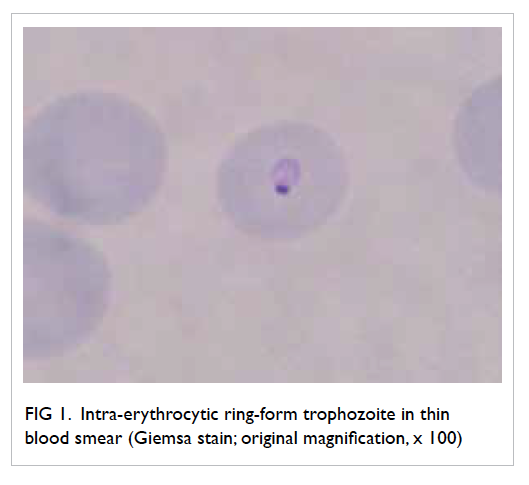
Figure 1. Intra-erythrocytic ring-form trophozoite in thin blood smear (Giemsa stain; original magnification, x 100)
He soon developed shock and was
transferred to the ICU. Further blood tests revealed
hyperbilirubinaemia and evidence of haemolysis.
Peripheral blood smear for malaria was repeated
and revealed 3.8% parasitised red cells. Pleomorphic
ring forms were noted, with occasional arrangement
in a cross-like pattern (Fig 2). Rapid antigen test for P falciparum was negative and this raised the
suspicion of babesiosis. A subsequent blood Babesia
microti polymerase chain reaction (PCR) test was
positive and a diagnosis of babesiosis was confirmed.
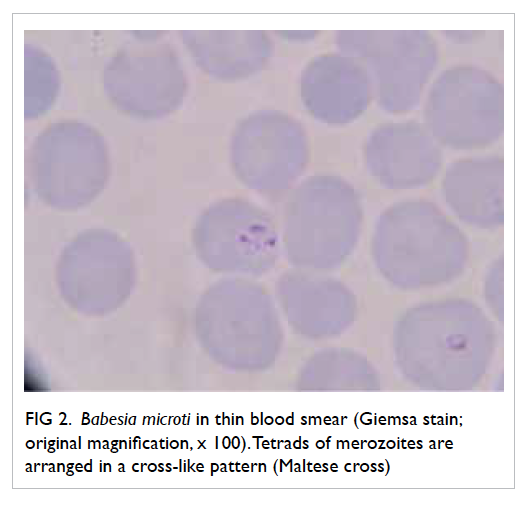
Figure 2. Babesia microti in thin blood smear (Giemsa stain; original magnification, x 100). Tetrads of merozoites are arranged in a cross-like pattern (Maltese cross)
Quinine and clindamycin were initiated. The
patient experienced severe tinnitus after 5 days of
treatment and quinine was stopped. Doxycycline
was added for treatment due to persistent fever
and because it is known that Lyme’s disease can be
a concurrent infection. He stayed in the ICU for 6
days and his condition was stabilised with resolution
of fever. Blood smear for Babesia turned negative on
day 14 of treatment. Supportive blood transfusion
was given for anaemia and the patient was discharged
after 21 days.
He attended regular follow-ups in our clinic.
Repeated blood smear for babesiosis and Babesia
PCR test (in September 2012, 3 months after
treatment) were negative. He had the basement of
his New York apartment professionally cleaned and
had his dog treated for ticks.
Discussion
This is the second imported case of human babesiosis
in Hong Kong since 2007.1 Babesiosis is a tick-borne
disease in which patients are infected with intra-erythrocytic
parasites of the genus Babesia. The
common species affecting humans are B microti and
Babesia divergens, mostly found in the United States
and Europe, respectively. White-footed mice are
the primary reservoir host, but other small rodents
may also carry B microti. The Babesia parasites are
transmitted to humans accidentally by Ixodid tick
bites. In the United States, Ixodes scapularis is the
most common vector. The ticks become infected
with B microti when they feed on vertebrate reservoir
hosts such as infected white-footed mice and white-tailed
deer. Humans are usually accidental hosts.2
The diagnosis of human babesiosis is made by
microscopic examination of Giemsa-stained thick
and thin blood smears. Microscopically, Babesia
species may appear as round or oval-shaped forms,
with blue cytoplasm and red chromatin. Multiple
parasites may be present in a single-infected red
blood cell. Differentiation of the ring-form of
Babesia species and P falciparum may be difficult.
Some distinguishing features of babesiosis include
the presence of extra-erythrocytic forms in severe
cases and the absence of pigment deposits typically
seen in older ring stages of P falciparum. The Maltese
cross pattern in which tetrads of merozoites are
arranged is pathognomonic of babesiosis, but they
are not commonly seen.3 Nonetheless, the different
species of babesiosis cannot be distinguished by
microscopic examination. Real-time PCR performed
on DNA targeting the 18S rRNA gene of B microti
is therefore a more specific and accurate method to
detect B microti.4
Our patient was a case of possible pet-associated
human Babesia infection. Babesiosis is a
zoonotic protozoan disease of medical, veterinary,
and economic importance. A case of human
babesiosis acquired from a pet dog has been reported
where the dog was heavily infested with ticks.5 In a
Brazilian evaluation of ectoparasites in dogs kept
in apartments, Ixodid nymphs were found in 2%.
In houses with grassy yards, 18% of pet dogs were
found to carry Ixodid nymphs.6 Different species of
Ixodid ticks have been identified in dogs, with the
head being the most common site of attachment.
The activity of tick attachment peaks in spring
and in autumn. Canine babesiosis is diagnosed by
veterinarians in approximately 20% of tick-infested
dogs.7 In one study in New York state, I scapularis
ticks were collected from recreational lands to
determine the prevalence and distribution of tick-borne
pathogens. The overall prevalence of B microti
was approximately 3% among both nymphs and
adult ticks.8
In Hong Kong, around 250 000 households
are estimated to keep dogs or cats, representing
10.6% of all households.9 There has been no single
reported case of human babesiosis in Hong Kong
although the disease is common in the cat and
dog population. Recently a new Babesia species,
Babesia hongkongensis has been identified in the
feline population.10 The prevalence of this new local
species is low among free-roaming cats in Hong
Kong and the pathogenicity in pet cats is unknown.
The clinical presentation of human babesiosis
varies. Infected patients may present with a mild-to-moderate viral-like illness, with gradual onset of
chills, sweats, headache, arthralgia, and anorexia.
Some patients present with a prolonged course of
pyrexia of unknown origin. Physical examination
may reveal mild splenomegaly or hepatomegaly.
Severe infection generally occurs in those with
an underlying immunosuppressed condition,
particularly patients with previous splenectomy.
Complications of babesiosis include acute respiratory
failure, disseminated intravascular coagulation,
and multi-organ failure.3
Combination therapy with atovaquone and
azithromycin is the treatment of choice for mild-to-moderate Babesia infection. In severe cases, the
use of clindamycin and quinine is recommended.
In general, the combination of atovaquone and
azithromycin is better tolerated with fewer adverse
effects.11 All doses of antimicrobial therapy
are administered for 7 to 10 days. For severely
immunocompromised patients with persistent
relapsing infection, a longer duration of at least 6
weeks’ treatment is recommended, continuing for
2 weeks after blood smears become negative for
Babesia. For fulminant cases, exchange transfusion
may be required.2 12
References
1. Wong WS, Chung JY, Wong KF. Images in haematology.
Human babesiosis. Br J Haematol 2008;140:364. Crossref
2. Vannier E, Krause PJ. Human babesiosis. N Engl J Med
2012;66:2397-407. Crossref
3. Vannier E, Gewurz BE, Krause PJ. Human babesiosis.
Infect Dis Clin North Am 2008;22:469-88, viii-ix. Crossref
4. Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci
S. A new real-time PCR assay for improved detection of
the parasite Babesia microti. J Clin Microbiol 2012;50:903-8. Crossref
5. EL-Bahnasawy MM, Khalil HH, Morsy TA. Babesiosis
in an Egyptian boy acquired from pet dog, and a general
review. J Egypt Soc Parasitol 2011;41:99-108.
6. Soares AO, Souza AD, Feliciano EA, Rodrigues AF,
D’Agosto M, Daemon E. Evaluation of ectoparasites and
hemoparasites in dogs kept in apartments and houses with
yards in the city of Juiz de Fora, Minas Gerais, Brazil [in
Portuguese]. Rev Bras Parasitol Vet 2006;15:13-6.
7. Földvári G, Farkas R. Ixodid tick species attaching to dogs
in Hungary. Vet Parasitol 2005;129:125-31. Crossref
8. Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee
J, Backenson PB. Prevalence of Borrelia burgdorferi
(Spirochaetales: Spirochaetaceae), Anaplasma
phagocytophilum (Rickettsiales: Anaplasmataceae), and
Babesia microti (Piroplasmida: Babesiidae) in Ixodes
scapularis (Acari: Ixodidae) collected from recreational
lands in the Hudson Valley Region, New York State. J Med
Entomol 2014;51:226-36. Crossref
9. Thematic Household Survey Report No. 48. Census and
Statistics Department, Hong Kong SAR Government 2011. Available from: http://www.statistics.gov.hk/pub/B11302482011XXXXB0100.pdf. Accessed Feb 2016.
10. Wong SS, Poon RW, Hui JJ, Yuen KY. Detection of Babesia
hongkongensis sp. nov. in a free-roaming Felis catus cat in
Hong Kong. J Clin Microbiol 2012;50:2799-803. Crossref
11. Krause PJ, Lepore T, Sikand VK, et al. Atovaquone and
azithromycin for the treatment of babesiosis. N Engl J Med
2000;343:1454-8. Crossref
12. Dorman SE, Cannon ME, Telford SR 3rd, Frank KM,
Churchill WH. Fulminant babesiosis treated with
clindamycin, quinine, and whole-blood exchange
transfusion. Transfusion 2000;40:375-80. Crossref

