Hong Kong Med J 2016 Aug;22(4):334–40 | Epub 3 Jun 2016
DOI: 10.12809/hkmj154673
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Managing malignant pleural effusion with an indwelling pleural catheter: factors associated with spontaneous pleurodesis
WM Wong, FHKCP, FHKAM (Medicine);
Terence CC Tam, FHKCP, FHKAM (Medicine);
Matthew KY Wong, MB, BS, FRCP;
Macy MS Lui, FHKCP, FHKAM (Medicine);
Mary SM Ip, MD, FRCP;
David CL Lam, MD, FRCP
Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr David CL Lam (dcllam@hku.hk)
Abstract
Introduction: Malignant pleural effusion can be
recurrent despite active anti-cancer treatment.
Significant malignant pleural effusion leads to
debilitating dyspnoea and worsening quality of life in
patients with advanced cancer. An indwelling pleural
catheter offers a novel means to manage recurrent
malignant pleural effusion and may remove the
need for repeated thoracocentesis. Spontaneous
pleurodesis is another unique advantage of
indwelling pleural catheter placement but the
factors associated with its occurrence are not clearly
established. The aims of this study were to explore
the safety of an indwelling pleural catheter in the
management of symptomatic recurrent malignant
pleural effusion, and to identify the factors associated
with spontaneous pleurodesis.
Methods: This case series with internal comparisons
was conducted in the Division of Respiratory
Medicine, Department of Medicine, Queen Mary
Hospital, Hong Kong. All patients who underwent
insertion of an indwelling pleural catheter from
the initiation of such service from January 2010 to
December 2014 were included for data analysis.
Patients were monitored until December 2014, with
the last catheter inserted in July 2014.
Results: Between 2010 and 2014, a total of 23
indwelling pleural catheters were inserted in 22
consecutive patients with malignant pleural effusion,
including 15 (65.2%) cases with malignant pleural
effusion as a result of metastatic lung cancer. Ten
(43.5%) cases achieved minimal output according to
defined criteria, in five of whom the pleural catheter
was removed without subsequent re-accumulation
of effusion (ie spontaneous pleurodesis). Factors
associated with minimal output were the absence
of trapped lung (P=0.036), shorter time from first
appearance of malignant pleural effusion to catheter
insertion (P=0.017), and longer time from catheter
insertion till patient’s death or end of study (P=0.007).
Conclusions: An indwelling pleural catheter provides
a safe means to manage symptomatic malignant
pleural effusion. Potential clinical factors associated
with minimal output were identified along with the
occurrence of spontaneous pleurodesis, which is
a unique advantage offered by indwelling pleural
catheter.
New knowledge added by this study
- An indwelling pleural catheter (IPC) offers a new and safe management option for symptomatic malignant pleural effusion (MPE).
- Potential clinical factors associated with spontaneous pleurodesis were identified.
- IPC is a safe management option for MPE.
- In addition to drainage of effusion, the use of an IPC may be followed by spontaneous pleurodesis that obviates the need for any additional chemical sclerosant.
Introduction
Malignant pleural effusion (MPE) develops in up
to 50% of patients with advanced lung cancer1 and
can also develop in metastatic pleural involvement
from non-pulmonary cancers. Such complication
can be recurrent despite active anti-cancer
treatment and thus difficult to manage.1 Significant
MPE leads to debilitating dyspnoea and worsening
quality of life in patients with terminal cancer.2
Conventional management options of MPE include
thoracocentesis, chest tube drainage, and chemical
and surgical pleurodesis.3 Nonetheless, MPE often
recurs and necessitates repeated thoracocentesis
or chest tube drainage.4 Chemical pleurodesis via
an intercostal chest tube may entail prolonged
hospitalisation and despite initial ‘success’, MPE
often recurs a few months later.5 Surgical pleurodesis
is often too invasive for frail cancer patients.6
Systemic anti-cancer treatment may reduce MPE but
there is no guarantee of success.7 To secure symptom
relief and to minimise repeated interventions and
hospitalisation in refractory MPE was a constant
challenge, until an indwelling pleural catheter (IPC)
became more commonly used.8
An IPC is intended to be left in situ in the
pleural cavity permanently in patients with advanced
cancer. Insertion is under local anaesthesia, and
supplemented with conscious sedation if needed.
An IPC is a silicon catheter with a polyester cuff for
anchoring the catheter at the subcutaneous tunnel
that serves to reduce infection. At the end of the
external portion of the catheter is a silicone valve
that remains closed unless connected to a designated
drainage line or vacuum bottle. Vacuum bottles are
not reusable and are discarded after each episode of
drainage. Patients are usually advised to have IPC
drainage every 1 or 2 days, especially when output
remains substantial. In addition, drainage should be
done whenever symptoms of MPE occur (Fig).
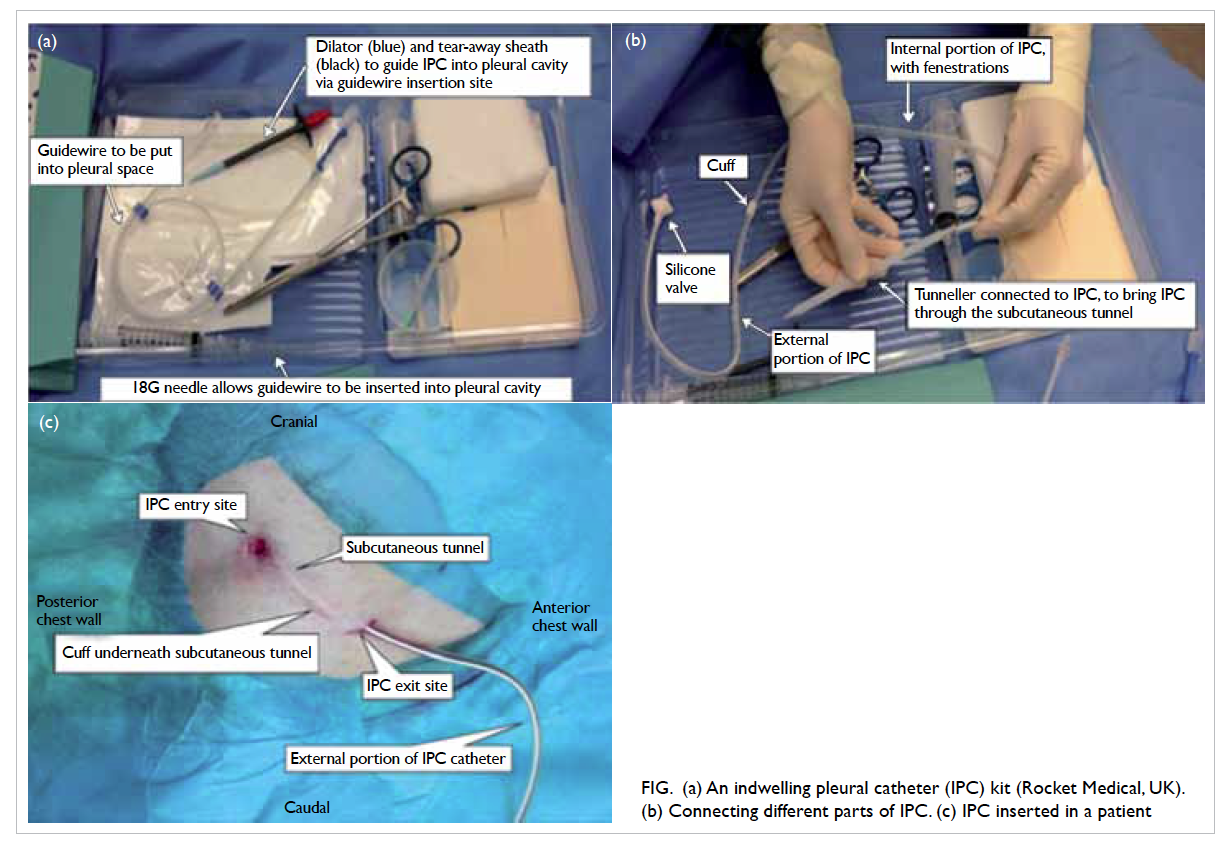
Figure. (a) An indwelling pleural catheter (IPC) kit (Rocket Medical, UK). (b) Connecting different parts of IPC. (c) IPC inserted in a patient
The guidelines for management of MPE
published by the British Thoracic Society suggest
that IPC is an alternative option for patients whose
estimated survival exceeds 1 month and who have
either a trapped lung or recurrent pleural effusion
following a trial of pleurodesis.3 First-line use
of IPC in patients who have no previous trial of
pleurodesis has also been shown to be superior to
talc pleurodesis with subjects being less dyspnoeic
at 6 months, and less likely to need further pleural
procedures, and reduced hospital stay by 3.5 days.9
Another prospective open-label trial that compared
IPC with talc slurry pleurodesis as first-line
treatment for MPE also demonstrated that first-line
use of IPC conferred non-inferior improvement
in dyspnoea and quality of life, reduced effusion-related hospital stay by 7 to 11 days, and required
less subsequent pleural procedures compared with
talc slurry pleurodesis.10 Research has shown that
IPC is a safe procedure, with no complications in
87.5% (range, 54.5-100%) of patients.11 Although
the IPC is designed to be left permanently in situ in
the pleural cavity in patients with advanced cancer,
one unique advantage of IPC is the occurrence of
autopleurodesis or spontaneous pleurodesis (SP)—ie pleurodesis achieved following IPC insertion
without the use of sclerosant. The achievement of
SP may enable consequent removal of the IPC. The
pooled rate of SP in MPE patients has been reported
to be 45.6%,11 achieved after a mean duration of 26
to 56 days after IPC insertion.11 12 13 14 15 16 17 18 19 20 The possibility of SP is attractive as there is a chance that an IPC will
no longer be required. The aims of this study were
to review our single-centre experience of the safety
of IPC in the management of symptomatic MPE and
to explore the potential clinical factors associated
with SP. To our knowledge, this is the first IPC study
published in Hong Kong.
Methods
All patients who underwent IPC insertion at the Division of Respiratory Medicine, Department of Medicine, Queen Mary Hospital since initiation of the IPC service in
January 2010 up to December 2014 were included
for data analysis. Patients and data were followed up
until December 2014, with the last IPC inserted in
July 2014. The study was approved by the University
of Hong Kong/Hong Kong Hospital Authority Hong
Kong West Cluster Institutional Review Board/Ethics Committee (HKU/HAHO HKWC IRB/EC
UW13-581) and informed consent was obtained
from patients.
An IPC was inserted in patients with MPE
who had trapped lung or prior failed pleurodesis or
persistent high effusion output from a chest drain
and a high chance of pleurodesis failure, or in patients
who preferred IPC as their first-line management of
MPE. The IPC kits (Rocket Medical, UK) were used
and IPCs were inserted in the endoscopy room under
local anaesthesia supplemented with conscious
sedation if needed.
The electronic patient records, in-patient
records, chest radiographs, and drainage diaries
were retrospectively reviewed. Data regarding
patient demographics, primary malignancy, cancer
treatment, history of thoracic irradiation, number
and type of prior pleural procedures, indications
for IPC, serum albumin level before IPC insertion,
laboratory analysis of pleural fluid obtained prior to
IPC insertion, and IPC-related complications and
admissions were collected and evaluated. ‘Massive
effusion’ was defined as more than two thirds of the
hemithorax. Effusion less than or equal to two thirds
of the hemithorax was defined as ‘non-massive effusion’.
Trapped lung was clinically diagnosed when chest
X-ray showed an incompletely re-expanded lung
despite adequate drainage and suction, together with
a compatible tumour status predisposing to trapped
lung (eg endobronchial tumour). The number of
IPCs inserted, instead of the number of patients,
was used for analysis in this study unless otherwise
specified.
Although IPC removal could be considered
when SP was achieved clinically, there were patients
who achieved minimal IPC output in whom IPC was
not removed due to other clinical considerations
or patient preference. Hence, the rate of SP would
be underestimated if only IPC removal of the basis
of minimal output was considered to reflect SP.
Therefore, in this study patients were deemed to have
achieved ‘minimal output’ if there was a persistently
reduced IPC output of ≤50 mL per day on average
that was not secondary to IPC complications, and
regardless of whether the IPC was removed or kept
in situ. Patients who persistently had an average
IPC output that exceeded 50 mL per day, or had
little output due to IPC complications (eg blocked
IPC or significant pleural loculation) were defined
as the ‘persistent output’ group. As achievement of
SP did not necessarily infer IPC removal, because of
patient preference and/or other considerations, the
endpoint ‘minimal output’ was used for analysis of
factors associated with SP.
The IBM PASW statistical software version 20
was used for data analysis. Association of clinical
factors with outcome was analysed with Fisher’s
exact test, independent sample t tests, and Mann-Whitney test where appropriate. Shapiro-Wilk
tests were used to check for normal distribution of
individual continuous variables. As minimal output
was a dichotomous variable, the point-biserial
correlation method was used for association analysis
between minimal output and other factors that were
continuous variables. The P values were two-sided
and were considered statistically significant if <0.05.
Results
A total of 23 IPCs were inserted in 22 consecutive
patients with symptomatic MPE. Insertion of 15
(65.2%) IPCs were in patients with MPE from
metastatic lung cancer. A further six were inserted
for MPE from metastatic breast cancer and two in
patients with MPE from metastatic colon cancer.
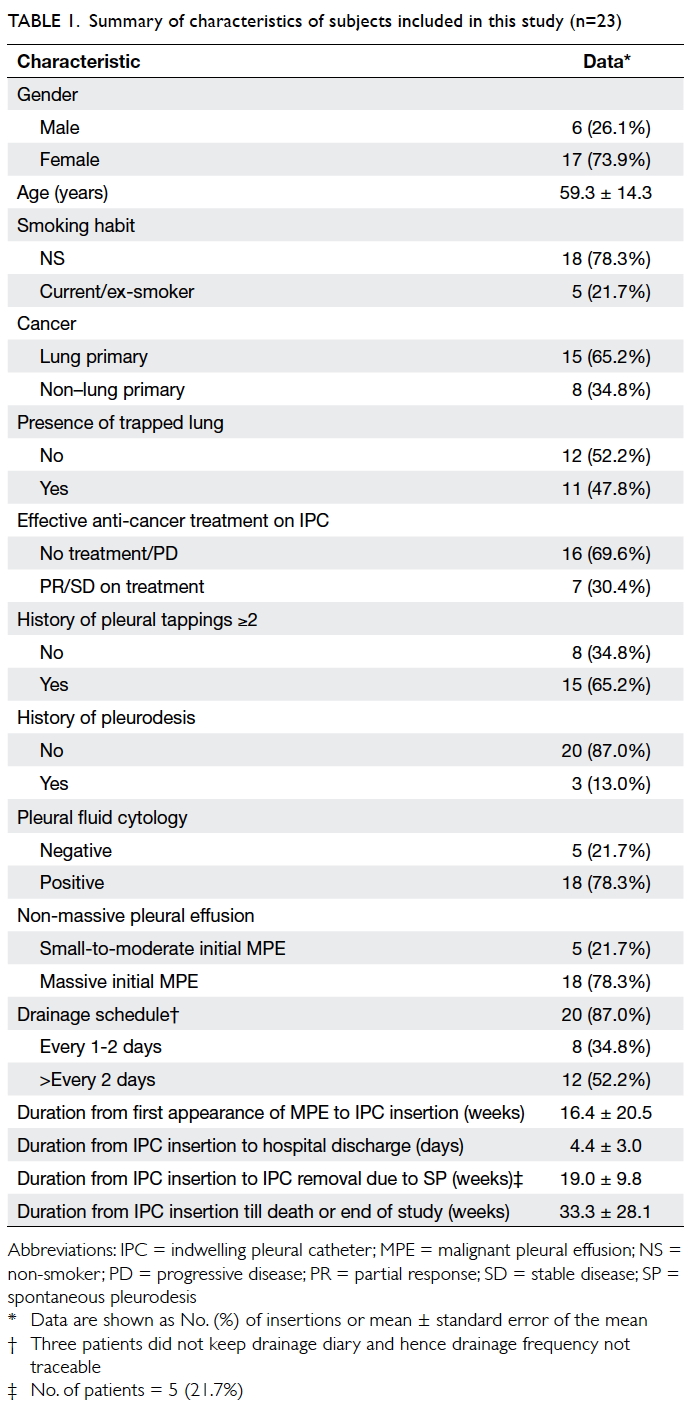
The characteristics of patients are shown in Table 1.
The mean (± standard error of the mean) duration of
follow-up was 33.3 ± 28.1 weeks.
Patients were admitted for symptomatic MPE
or elective IPC insertion. Patients were able to be
discharged with a mean of 4 days following IPC insertion.
Ambulatory IPC drainage via vacuum bottles was
performed by patients and/or their carers, except
one patient who was attended by outreach nurses of
the palliative care team.
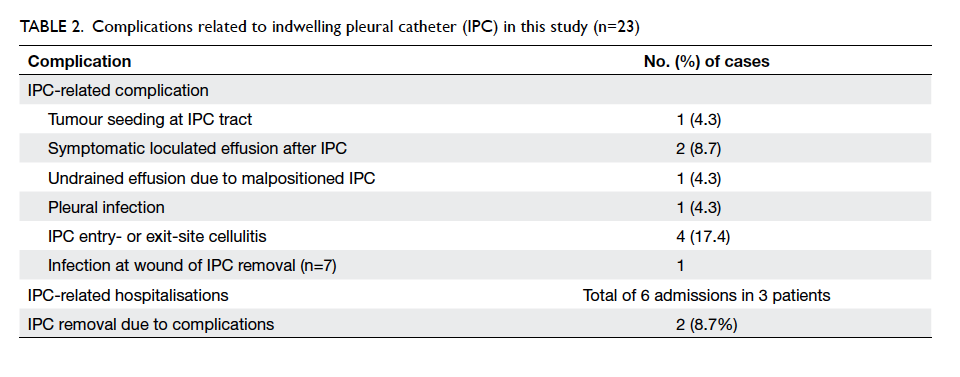
Complications related to IPC occurred in 10
(43.5%) cases (Table 2). Site infection and wound
infection following IPC removal were minor and all
resolved after a course of oral antibiotics without
the need for hospitalisation. Tumour seeding at the IPC
tract was successfully treated by local radiotherapy.
Two patients had symptomatic loculated effusion
following IPC insertion and required intrapleural
fibrinolytics: only one of them improved.
Complications necessitated removal of two IPCs.
One patient developed empyema 6 months after
IPC insertion. Pseudomonas aeruginosa was
persistently isolated from pleural fluid despite
appropriate antibiotics; infection resolved following
IPC removal. Another patient developed intractable
cough and it was suspected that her IPC was trapped
at the right oblique fissure causing irritation. Cough
improved following IPC removal. There were six
IPC complication–related hospitalisations (either
clinical or emergency admissions) in three patients:
the two patients with symptomatic loculations on
the IPC requiring fibrinolytics and the patient with
empyema mentioned above.
A total of 10 patients achieved minimal output: IPC was removed in five (21.7%) without subsequent effusion re-accumulation and the other five patients
achieved minimal output but retained their IPC. In
another two patients, IPC was removed because of
complications as mentioned before. No difficulties
were encountered during any IPC removal.
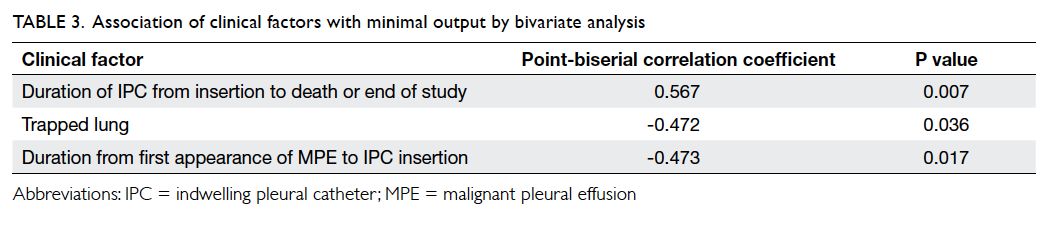
Significant factors associated with minimal
output were the absence of trapped lung (P=0.036),
shorter time from first appearance of MPE to IPC
insertion (24.5 ± 24.2 weeks in persistent output
group vs 5.75 ± 4.91 weeks in minimal output group;
P=0.017), and longer time from IPC insertion till
patient’s death or end of study (whichever was
earlier; 20.2 ± 19.5 weeks in persistent output group vs 50.3
± 29.2 weeks in minimal output group; P=0.007;
Table 3).
Discussion
In this small series of 22 patients with 23 IPCs,
mainly minor complications were encountered.
A serious IPC complication, namely empyema,
occurred in one (4.3%) case who was successfully
treated with antibiotics and removal of IPC without
serious consequences. Insertion of IPC is considered
a relatively safe procedure: up to 87.5% (range,
54.5-100%) of patients have no complications
following the insertion.11 Complications reported
in the literature include local pain (0.4-13%),
bleeding (0-0.9%), pneumothorax (0-38%), cellulitis
at exit site (1.3-25%), pleural infection (0-16.7%),
asymptomatic loculations (4-7.3%), symptomatic
loculations (2-13.5%), IPC tract metastasis (0-13.6%),
clogged catheter (0-17.6%), IPC dislodgement
(1.3-17.7%), and fractured IPC during removal
(9.8%). Previous studies suggest that up to 20.6%
(range, 1.6-20.6%) of IPCs need to be removed due
to complications.9 10 11 14 15 21 22 Nonetheless, serious
complications are uncommon; the most common
being pleural infection (0-16.7%).23 The TIME2
study reported that the risk of pleural infection was
13.4% in the IPC group compared with 1.9% in the
talc slurry pleurodesis group.9 Chemotherapy is
not regarded as a contra-indication to IPC, or vice
versa. No increased risk of pleural infection has been
observed in patients who receive chemotherapy with
an IPC in situ.24 Symptomatic loculations following
IPC insertion is another relatively significant
complication, as they often necessitate admission
for management such as intrapleural fibrinolysis or
other pleural procedure.
When the daily IPC output reduces to a
certain level (the exact ‘amount’ remains arbitrary),
IPC removal can be considered and SP is achieved
if there is no significant re-accumulation following
IPC removal. In reality, some patients had little
IPC output but the catheter was left in situ due to
other clinical considerations. The rate of SP could
be underestimated if it was solely reflected by the
ultimate rate of IPC removal, hence ‘minimal output’
was used in this study as the surrogate of SP during
analysis of factors that contributed to SP.
We determined that absence of trapped lung,
shorter time from first appearance of MPE to IPC
insertion, and longer time from IPC insertion till
patient’s death or end of study were associated with
minimal output. Trapped lung unsurprisingly led to
a higher chance of persistent output. Nonetheless, it
has been observed that patients with IPC inserted
for trapped lung can still achieve SP,12 15 17 18 20 or their lung expansion will improve after IPC.17 In
our cohort, two patients had their trapped lung re-expanded
after IPC insertion; one of whom had IPC
removed successfully without re-accumulation of
effusion.
It appears from this study that a shorter time
from MPE to IPC insertion could be associated with
the achievement of a minimal output state. This
could imply that the earlier an IPC is inserted, the
better chance of achieving minimal output or even
SP. Both a history of multiple pleural procedures
(which was arbitrarily defined in this study as
requiring two or more episodes of pleurocentesis or
chest drainage) and a history of failed pleurodesis
were usually indicative of refractory or difficult-to-manage MPE.25 It has never been ascertained
whether earlier IPC insertion rather than repeated
attempts at pleurocentesis or pleurodesis will
increase the chance of SP with IPC. Both factors were
not significantly associated with minimal output in
our small cohort. Further studies are required to
investigate whether prompt insertion of IPC as soon
as possible after development of MPE will improve
the likelihood of SP.
Patients who achieved minimal output had a
longer time from IPC insertion until death or end
of study (20.2 ± 19.5 weeks in the persistent output group
vs 50.3 ± 29.2 weeks in the minimal output group;
P=0.007). Minimal output may be a marker of overall
disease control. Lung cancer was the underlying
pathology in eight of the 10 subjects who achieved
minimal output, of whom six had adenocarcinoma
and were prescribed targeted therapy and
chemotherapy. Whether the concomitant use
of anti-cancer treatments for these lung cancer
patients contributed to longer survival following
IPC insertion could not be established from this
small cohort of lung cancer patients. Comparison
with non–lung cancer patients with IPC in this
study could not be made as patients with metastatic
breast or colorectal tumour with MPE had different
treatment strategies. As at December 2014, only four
of the 22 patients were still living. They were patients
with adenocarcinoma of the lung on palliative
chemotherapy/tyrosine kinase inhibitors. Among
these four patients, one had her IPC removed earlier
due to SP achievement, two had IPC removed earlier
due to IPC-related complications, and one still had
IPC in situ with persistent output.
Minimal output was used as a surrogate of SP
in this study rather than actual IPC removal in the
hope that it would better reflect what clinical factors
contribute to SP. Comparison of time from IPC
insertion to minimal output achievement in those
five patients whose IPCs were ultimately removed
and the five patients in whom IPC remained in
situ despite minimal output revealed no significant
difference (30 [interquartile range, 15-59] days vs 23
[standard error of the mean, 6.63] days). Nonetheless,
one must not ignore the reasons for non-removal of
IPC despite minimal output since they impact the
ultimate goal of IPC removal. In this study, there
were five patients who achieved minimal output
but in whom IPCs remained in situ due to various
reasons: poor performance state and short life
expectancy, undergoing cycles of chemotherapy, or
patient preferences.
This study was limited by the very small
sample size and its retrospective nature. There were
missing data and the dichotomous groupings, eg IPC
drainage every 1 to 2 days versus less frequent, were
crude and arbitrary. For example, more-frequent IPC
drainage to increase the chance of pleural apposition
may theoretically increase the chance of SP, although
in this study IPC drainage every 1 to 2 days versus less
frequent was not associated with minimal output.
This could be related to the crude grouping of the
IPC drainage frequency due to the retrospective
design of this study that did not allow us to properly
allocate the IPC drainage schedule. Further studies
to identify modifiable clinical factors that may
facilitate SP would be particularly meaningful.
Conclusions
Insertion of IPC was shown to be a safe technique
in the management of symptomatic MPE. Potential
factors associated with minimal output, which may
predict SP, were absence of trapped lung, shorter time
from first appearance of MPE to IPC insertion, and
longer time with IPC. Validation by further studies
is required owing to the small number of subjects
in this study. More data are needed regarding
modifiable factors that contribute to achievement of
minimal output, as the removal of IPC offers further
enhancement of quality of life.
Acknowledgement
The authors would like to thank Ms Crystal Kwan for
assistance in statistical analysis.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Shaw P, Agarwal R. Pleurodesis for malignant pleural
effusions. Cochrane Database Syst Rev 2004;(1):CD002916. Crossref
2. Lorenzo MJ, Modesto M, Pérez J, et al. Quality-of-Life
assessment in malignant pleural effusion treated with
indwelling pleural catheter: a prospective study. Palliat
Med 2014;28:326-34. Crossref
3. Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ;
BTS Pleural Disease Guideline Group. Management of a
malignant pleural effusion: British Thoracic Society Pleural
Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii32-40. Crossref
4. de Andrade FM. The role of indwelling pleural catheter in
management of malignant pleural effusion: A creative new
technique for an old method. Lung India 2015;32:81-2.
5. Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF,
Rahman NM. Comparing cost of indwelling pleural
catheter vs talc pleurodesis for malignant pleural effusion.
Chest 2014;146:991-1000. Crossref
6. Bhatnagar R, Kahan BC, Morley AJ, et al. The efficacy of
indwelling pleural catheter placement versus placement
plus talc sclerosant in patients with malignant pleural
effusions managed exclusively as outpatients (IPC-PLUS):
study protocol for a randomised controlled trial. Trials
2015;16:48. Crossref
7. Massarelli E, Onn A, Marom EM, et al. Vandetanib and
indwelling pleural catheter for non–small-cell lung cancer
with recurrent malignant pleural effusion. Clin Lung
Cancer 2014;15:379-86. Crossref
8. Fysh ET, Thomas R, Read CA, et al. Protocol of the
Australasian Malignant Pleural Effusion (AMPLE) trial:
a multicentre randomised study comparing indwelling
pleural catheter versus talc pleurodesis. BMJ Open
2014;4:e006757. Crossref
9. Davies HE, Mishra EK, Kahan BC, et al. Effect of an
indwelling pleural catheter vs chest tube and talc
pleurodesis for relieving dyspnea in patients with malignant
pleural effusion: the TIME2 randomized controlled trial.
JAMA 2012;307:2383-9. Crossref
10. Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural
catheters reduce inpatient days over pleurodesis for
malignant pleural effusion. Chest 2012;142:394-400. Crossref
11. Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety
of tunneled pleural catheters in adults with malignant
pleural effusions: a systematic review. J Gen Intern Med
2011;26:70-6. Crossref
12. Warren WH, Kalimi R, Khodadadian LM, Kim AW.
Management of malignant pleural effusions using the
Pleurx catheter. Ann Thorac Surg 2008;85:1049-55. Crossref
13. Warren WH, Kim AW, Liptay MJ. Identification of clinical
factors predicting Pleurx catheter removal in patients
treated for malignant pleural effusion. Eur J Cardiothorac
Surg 2008;33:89-94. Crossref
14. Sioris T, Sihvo E, Salo J, Räsänen J, Knuuttila A. Long-term
indwelling pleural catheter (PleurX) for malignant
pleural effusion unsuitable for talc pleurodesis. Eur J Surg
Oncol 2009;35:546-51. Crossref
15. Tremblay A, Michaud G. Single-center experience with
250 tunnelled pleural catheter insertions for malignant
pleural effusion. Chest 2006;129:362-8. Crossref
16. Bertolaccini L, Viti A, Gorla A, Terzi A. Home-management
of malignant pleural effusion with an
indwelling pleural catheter: ten years experience. Eur J Surg
Oncol 2012;38:1161-4. Crossref
17. Schneider T, Reimer P, Storz K, et al. Recurrent pleural
effusion: who benefits from a tunneled pleural catheter?
Thorac Cardiovasc Surg 2009;57:42-6. Crossref
18. Al-Halfawy A, Light R. Safety and efficacy of using a
surgivac pump for the drainage of chronic indwelling
pleural catheters in malignant pleural effusions.
Respirology 2008;13:461-4. Crossref
19. Bazerbashi S, Villaquiran J, Awan MY, Unsworth-White
MJ, Rahamim J, Marchbank A. Ambulatory intercostal
drainage for the management of malignant pleural effusion:
a single center experience. Ann Surg Oncol 2009;16:3482-7. Crossref
20. Ohm C, Park D, Vogen M, et al. Use of an indwelling pleural
catheter compared with thoracoscopic talc pleurodesis in
the management of malignant pleural effusions. Am Surg
2003;69:198-202.
21. Tremblay A, Mason C, Michaud G. Use of tunnelled
catheters for malignant pleural effusions in patients fit for
pleurodesis. Eur Respir J 2007;30:759-62. Crossref
22. Fysh ET, Wrightson JM, Lee YC, Rahman NM. Fractured
indwelling pleural catheters. Chest 2012;141:1090-4. Crossref
23. Gilbert CR, Lee HJ, Akulian JA, et al. A quality
improvement intervention to reduce indwelling tunneled
pleural catheter infection rates. Ann Am Thorac Soc
2015;12:847-53. Crossref
24. Mekhaiel E, Kashyap R, Mullon JJ, Maldonado F. Infections
associated with tunnelled indwelling pleural catheters in
patients undergoing chemotherapy. J Bronchology Interv
Pulmonol 2013;20:299-303. Crossref
25. Fysh ET, Bielsa S, Budgeon CA, et al. Predictors of
clinical use of pleurodesis and/or indwelling pleural
catheter therapy for malignant pleural effusion. Chest
2015;147:1629-34. Crossref