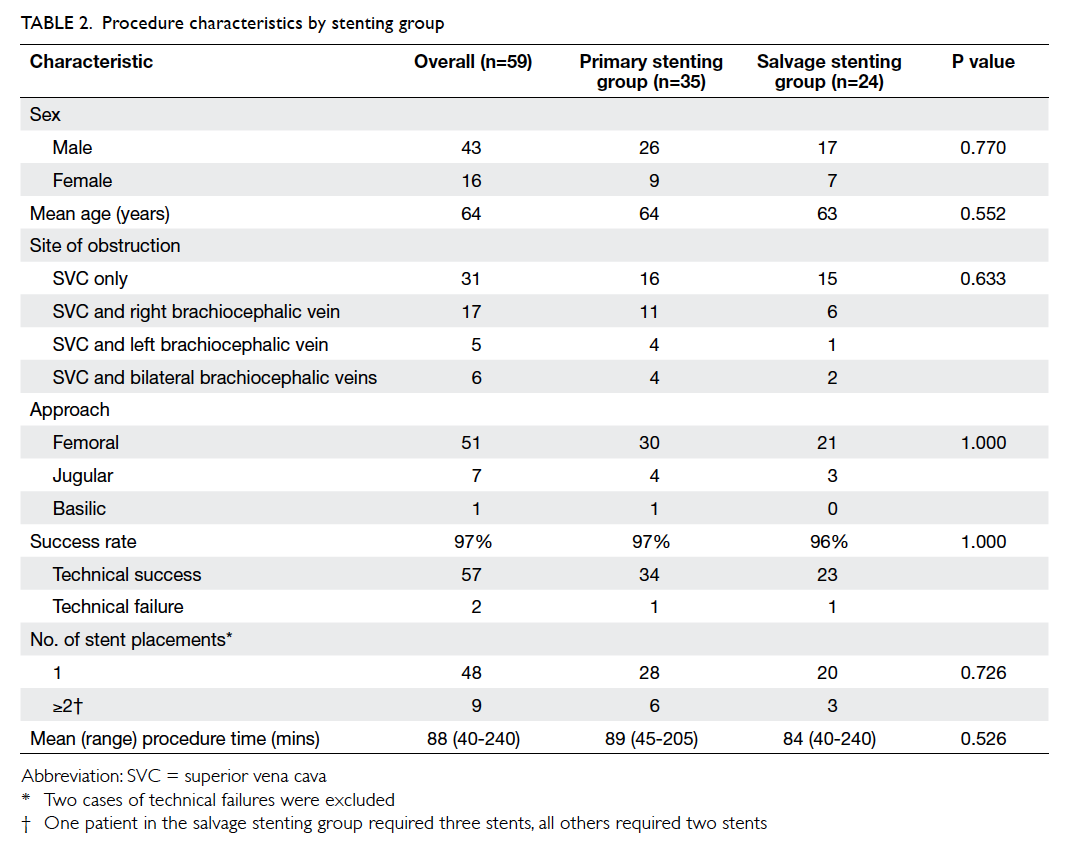
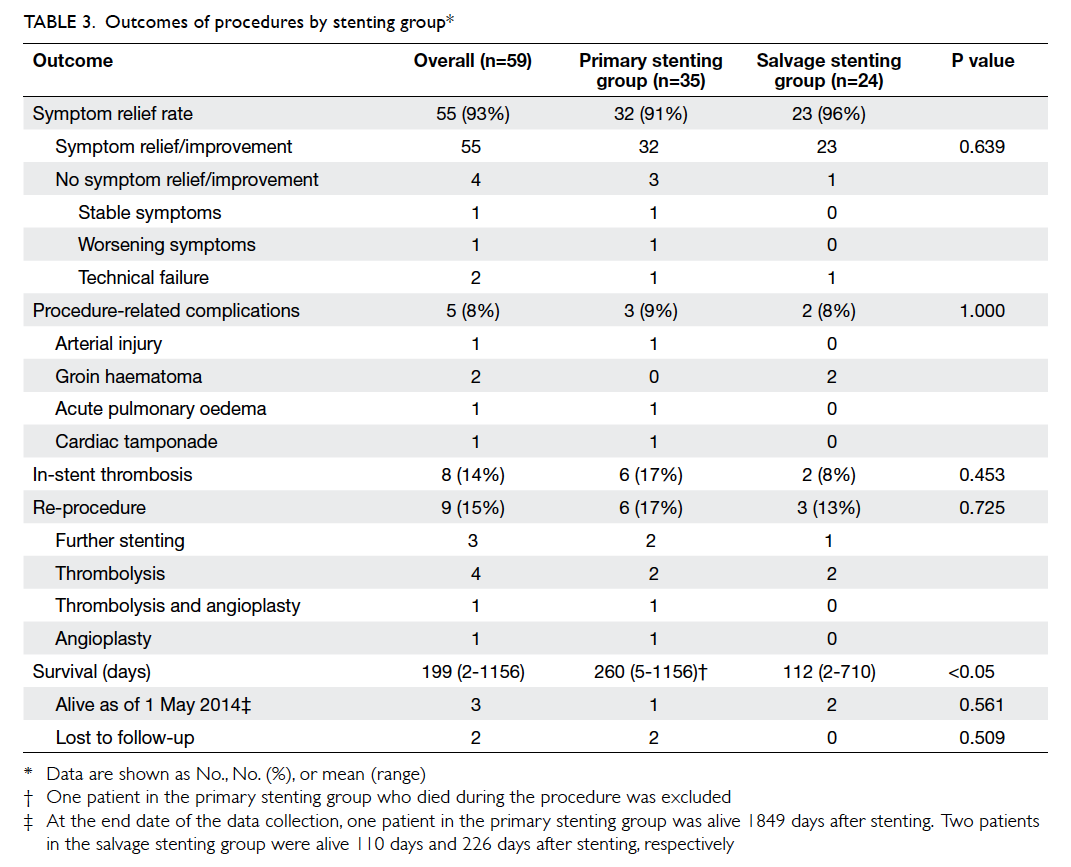
Hong Kong Med J 2015 Oct;21(5):426–34 | Epub 3 Jul 2015
DOI: 10.12809/hkmj144363
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Endovascular stenting in the management of
malignant superior vena cava obstruction:
comparing safety, effectiveness, and outcomes
between primary stenting and salvage stenting
ST Leung, MB, BS, FRCR;
Tony HT Sung, FRCR, FHKAM (Radiology);
Alvin YH Wan, FRCR, FHKAM (Radiology);
KW Leung, FRCR, FHKAM (Radiology);
WK Kan, FRCR, FHKAM (Radiology)
Department of Radiology, Pamela Youde Nethersole Eastern Hospital,
Chai Wan, Hong Kong
Corresponding author: Dr ST Leung (baryleung@hotmail.com)
Abstract
Objective: To compare the safety, effectiveness, and
outcomes of primary stenting and salvage stenting
for malignant superior vena cava obstruction.
Design: Case series with internal comparison.
Setting: Regional hospital, Hong Kong.
Patients: A total of 56 patients with malignant
superior vena cava obstruction underwent 59
stentings from 1 May 1999 to 31 January 2014. Patients’ characteristics, procedural details,
and outcomes were retrospectively reviewed. Of
the 56 patients, 33 had primary stenting before
conventional therapy and 23 had salvage stenting
after failure of conventional therapy. Statistical
analyses were made by Fisher’s exact test and Mann-Whitney U test.
Results: Primary lung carcinoma was the most
common cause of malignant superior vena cava obstruction (primary stenting, 22 patients;
salvage stenting, 16 patients; P=0.768), followed
by metastatic lymphadenopathy. Most patients
had superior vena cava obstruction only (primary
stenting, 16 patients; salvage stenting, 15
patients; P=0.633), followed by additional right
brachiocephalic vein involvement. Wallstents
(Boston Scientific, Natick [MA], US) were used in
all patients. Technical success was achieved in all
but two patients, one in each group (P=1.000). Only
one stent placement was required in most patients
(primary stenting, 28 patients; salvage stenting, 20
patients; P=0.726). Procedure time was comparable
in both groups (mean time: primary stenting,
89 minutes; salvage stenting, 84 minutes;
P=0.526). Symptomatic relief was achieved in most
patients (primary stenting, 32 patients; salvage
stenting, 23 patients; P=0.639). In-stent restenosis
and bleeding were the commonest complications
(primary stenting, 6 and 1 patients, respectively;
salvage stenting, 2 and 2 patients, respectively). Nine
patients required further treatment for symptom
recurrence (primary stenting, 6 patients; salvage
stenting, 3 patients; P=0.725).
Conclusion: Endovascular stenting is safe and
effective for relieving malignant superior vena cava
obstruction. No statistically significant differences
in number of stents, success rates, procedure times,
symptom relief rates, complication rates, and re-procedure
rates were found between primary
stenting and salvage stenting.
New knowledge added by this study
- Endovascular stenting is safe and effective for relieving malignant superior vena cava obstruction (SVCO) in both primary stenting and salvage stenting settings.
- Direct comparison between primary stenting and salvage stenting for safety, effectiveness, and outcomes of superior vena cava (SVC) stenting showed no significant differences in number of stents required, success rates, procedure times, symptom relief rates, complication rates, and re-procedure rates between the two groups.
- Primary SVC stenting should be considered for patients at their initial presentation with SVCO before conventional therapy by radiotherapy and/or chemotherapy.
- Salvage SVC stenting remains a safe and effective treatment for patients with SVCO after failure of radiotherapy and/or chemotherapy.
Introduction
Superior vena cava (SVC) syndrome encompasses
a constellation of symptoms and signs secondary
to superior vena cava obstruction (SVCO). The
syndrome frequently occurs secondary to extrinsic
SVC compression, mostly from malignant causes,
due to its low internal venous pressure and location
within the rigid structures in the mediastinum. The
resulting elevated venous pressure in the upper
body causes oedema of the head, neck, and upper
extremities. Oedema in the airway may cause life-threatening
airway obstruction, and cerebral oedema
may result in confusion and coma. There is also
decreased venous return causing haemodynamic
compromise. These all result in the significant
morbidity and mortality associated with SVCO.1 2
Since its first description by Charnsangavej
et al in 1986,3 SVC stenting has gained increasing
popularity in the management of SVCO due to its
rapid and effective relief of symptoms compared
with conventional therapy by radiotherapy and
chemotherapy. A systematic review by Rowell
and Gleeson4 concluded that stenting is the most
effective and rapid treatment for relieving SVCO
symptoms, providing overall symptomatic relief in
95% of patients with an 11% symptom recurrence
rate. Radiotherapy and chemotherapy, however,
could only achieve symptomatic relief in 60% to
77% of patients, with 17% to 19% of patients having
symptom recurrence.4
Stenting of SVC is traditionally offered as a
salvage therapy after failure of conventional therapy.
In recent years, an increasing number of hospitals
have begun to consider primary stenting as a first-line
treatment prior to conventional therapy due to
its promising results.2 5 However, there is currently a lack of studies directly comparing the results of
primary stenting before conventional therapy and
salvage stenting after failure of conventional therapy.
In addition, previous studies evaluating SVC stenting
are often limited by a small sample size and lack of
long-term follow-up. Only a few case series of
more than 50 patients are currently available in the
literature.6 7 8 9 10 11
With the aim of comparing the safety,
effectiveness, and outcomes between patients
undergoing primary stenting before conventional
therapy and salvage stenting after failure of
conventional therapy, we report our 15 years’
experience in the management of malignant SVCO
with Wallstent endoprosthesis (Boston Scientific,
Natick [MA], US).
Methods
A retrospective review of the indications, clinical
characteristics, procedures, complications, and
outcomes was performed for all patients with clinical
symptoms of SVCO who underwent SVC stenting at
a single hospital in Hong Kong from 1 May 1999 to
31 January 2014. Patients were identified from the
departmental internal records and the radiology
information system. All patients had computed
tomography performed prior to stent placement,
which revealed unresectable malignant SVCO. Patients’ medical and procedural records
were retrospectively reviewed by a radiologist who
was a Fellow of the Royal College of Radiologists with
subspecialty training in interventional radiology,
and who was blinded to whether the patient was
receiving primary stenting or salvage stenting
during the review of patients’ outcomes. The follow-up
period was considered as being from the day of
the procedure to the day of the latest information
or death, with the end of data collection fixed on
1 May 2014. This study was approved by the local
Institutional Review Board.
Patients were categorised into either the
primary stenting group or the salvage stenting group.
Patients in the primary stenting group had SVC
stenting performed at initial presentation of SVCO
before any radiotherapy and/or chemotherapy.
Patients in the salvage stenting group had SVC
stenting performed after failure of radiotherapy
and/or chemotherapy, which was defined as newly
developed or worsening SVCO symptoms despite
the use of radiotherapy and/or chemotherapy. The
primary stenting group comprised 33 patients with
35 SVC stentings done and the salvage stenting
group comprised 23 patients with 24 SVC stentings
performed.
Stent placement was performed under
local anaesthesia in an angiography suite with
cardiopulmonary monitoring for all patients after
obtaining informed consent. Pre-procedure superior
vena cavograms were performed for assessment of
site, length, degree of stenosis, and planning of stent
placement. Wallstent endoprostheses were used in
all patients. Intravenous heparin was administered
before stent placement.
The stenoses were first negotiated with a
guidewire. Placements of Wallstents across the
stenoses were then performed. Balloon angioplasty
was performed before and/or after stent placement
if considered necessary by the performing
interventional radiologist. Stent position and
patency were confirmed by post-procedural superior
vena cavogram, which also excluded any venous
rupture (Fig 1).
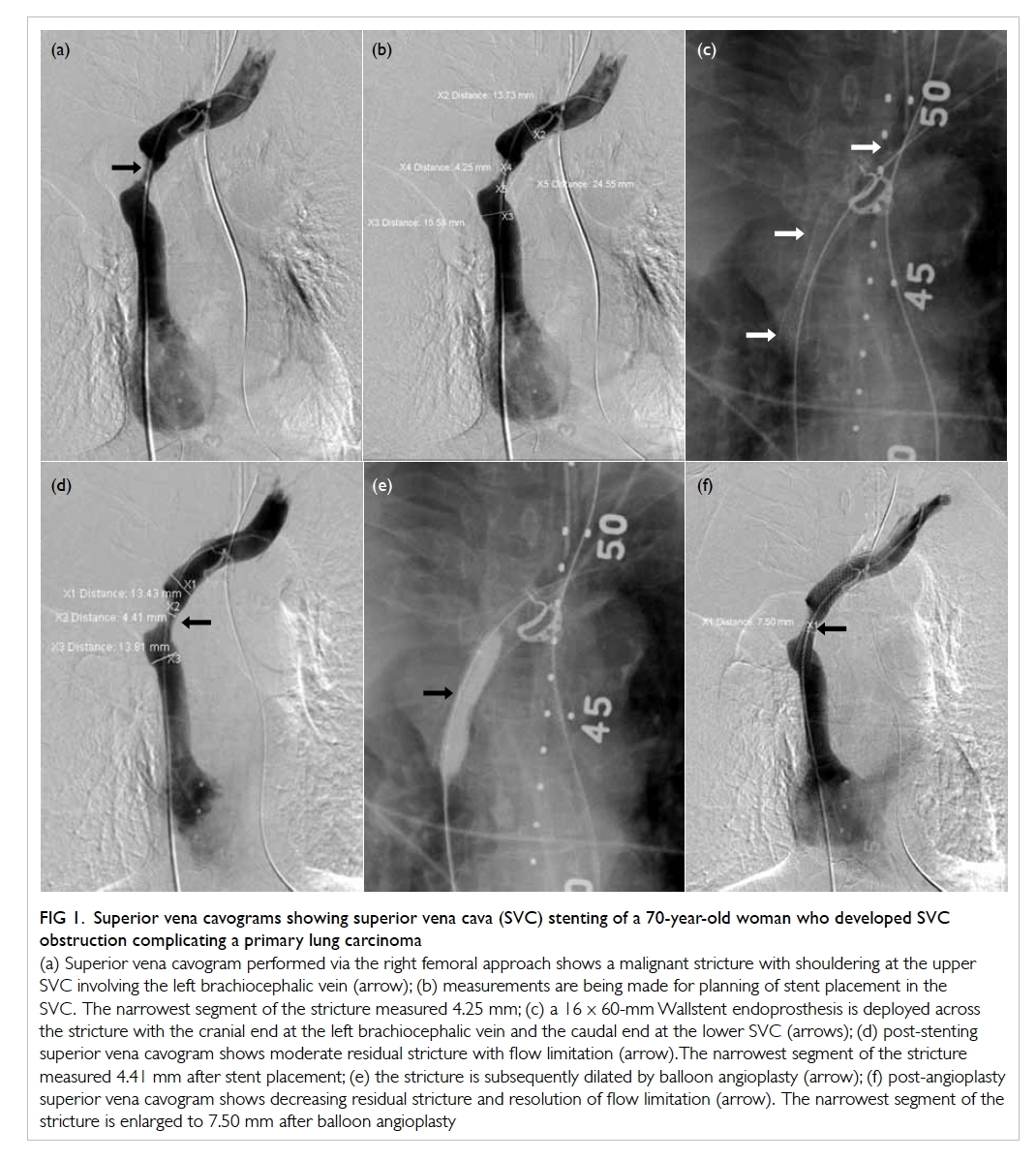
Figure 1. Superior vena cavograms showing superior vena cava (SVC) stenting of a 70-year-old woman who developed SVC obstruction complicating a primary lung carcinoma
(a) Superior vena cavogram performed via the right femoral approach shows a malignant stricture with shouldering at the upper SVC involving the left brachiocephalic vein (arrow); (b) measurements are being made for planning of stent placement in the SVC. The narrowest segment of the stricture measured 4.25 mm; (c) a 16 x 60-mm Wallstent endoprosthesis is deployed across the stricture with the cranial end at the left brachiocephalic vein and the caudal end at the lower SVC (arrows); (d) post-stenting superior vena cavogram shows moderate residual stricture with flow limitation (arrow). The narrowest segment of the stricture measured 4.41 mm after stent placement; (e) the stricture is subsequently dilated by balloon angioplasty (arrow); (f) post-angioplasty superior vena cavogram shows decreasing residual stricture and resolution of flow limitation (arrow). The narrowest segment of the stricture is enlarged to 7.50 mm after balloon angioplasty
Statistical analyses were performed by the Statistical Package for the Social Sciences
(Windows version 19.0; SPSS Inc, Chicago [IL],
US). P values were calculated by Fisher’s exact test
and Mann-Whitney U test when appropriate, and a
significance level of 0.05 was used.
Results
A total of 56 (40 male and 16 female) patients
underwent 59 SVC stentings for malignant SVCO
during the study period. All patients were included
in the study and their mean age was 64
years (range, 48-83 years). There were no statistically
significant differences in male-to-female ratio
(P=0.797), patient age (P=0.548), and underlying
causes between the primary and salvage stenting
groups. The background demographics of the two
groups of patients are summarised in Table 1.
Underlying cause
Primary lung carcinoma was the most common
cause in both groups of patients, accounting for
67% (n=22) in the primary stenting group and 70%
(n=16) in the salvage stenting group. No statistically
significant difference was seen between the two
groups (P=0.768).
Among the causes other than primary lung
carcinoma, metastatic lymphadenopathy was the
most common indication, which was seen in two
patients in the primary stenting group and four in
the salvage stenting group. Carcinoma of the breast
was the most common primary site, accounting
for three of the six patients. Other causes included
lymphoma (n=1), malignant thymic tumour (n=1),
and neuroendocrine tumour (n=1) [Table 1].
Site of obstruction
Among the 59 stenting procedures, obstruction at the
level of SVC only was most commonly encountered,
accounting for 46% (n=16) of cases in the primary
stenting group and 63% (n=15) of cases in the salvage
stenting group. Additional sites of obstruction were
found at the right brachiocephalic vein, bilateral
brachiocephalic veins, and left brachiocephalic vein.
There were no statistically significant differences
between the two groups (P=0.633) [Table 2].
Procedures
Results of the procedures are summarised in Table
2. No statistically significant differences were seen
between the two groups. The femoral approach was
used for most patients: 86% (n=30) in the primary stenting group and 88% (n=21) in the salvage stenting group. The jugular and basilic
approaches were used for the remaining patients.
Successful stent placement was achieved in all
but two patients, with similar success rates in both
groups of patients: 97% in the primary stenting group
and 96% in the salvage stenting group (P=1.000). One
failure occurred in the primary stenting group due to
development of fatal haemopericardium during the
procedure. Another failure occurred in the salvage
stenting group due to failure of stent placement
across the obstruction.
A single stent was sufficient to restore
vessel patency in most patients, with the results
comparable for both groups of patients: 82% (n=28)
in the primary stenting group and 87% (n=20)
in the salvage stenting group. No statistically
significant differences were seen between the two
groups (P=0.726). The remaining patients required
placement of two to three stents to alleviate the
obstruction. Anticoagulation following stent
placement was recommended for prevention of in-stent
thrombosis with the individual anticoagulation
regimen decided by the senior physicians and
oncologists.
The procedure times for the two groups of
patients showed no statistically significant difference
(P=0.526). The mean procedure time was 89 minutes
(range, 45-205 minutes) in the primary stenting
group, and 84 minutes (range, 40-240 minutes) in
the salvage stenting group (Table 2).
Treatment outcome
Table 3 summarises the outcomes after SVC
stenting. Resolution or improvement of symptoms
within 72 hours post-stenting was demonstrated in
most patients: 91% (n=32) in the primary stenting
group and 96% (n=23) in the salvage stenting group
(P=0.639). One patient in the primary stenting
group had worsening symptoms after stenting due
to development of in-stent thrombosis shortly after
stent placement.
Complications
Procedure-related complications were uncommon
and there were no statistically significant differences
between the two groups of patients for complication
rates: 9% (n=3) in the primary stenting group and 8%
(n=2) in the salvage stenting group (P=1.000). The
complications included haemopericardium (n=1),
acute pulmonary oedema (n=1), and bleeding-related
complications (groin haematoma, n=2;
arterial injury, n=1). One periprocedural death
occurred due to fatal haemopericardium and the
overall mortality was 1.7%.
For stent-related complications, in-stent
thrombosis was seen in 14% of patients: 17% (n=6)
in the primary stenting group and 8% (n=2) in the
salvage stenting group (P=0.453). No stent migration
was identified.
Patient outcomes
Following successful stent placement, a minority
of patients had recurrence of SVCO symptoms
requiring further interventions, including further
stenting, thrombolysis, and angioplasty (Fig 2).
Comparable results were seen in the two groups of
patients: 17% (n=6) in the primary stenting group and
13% (n=3) in the salvage stenting group (P=0.725).
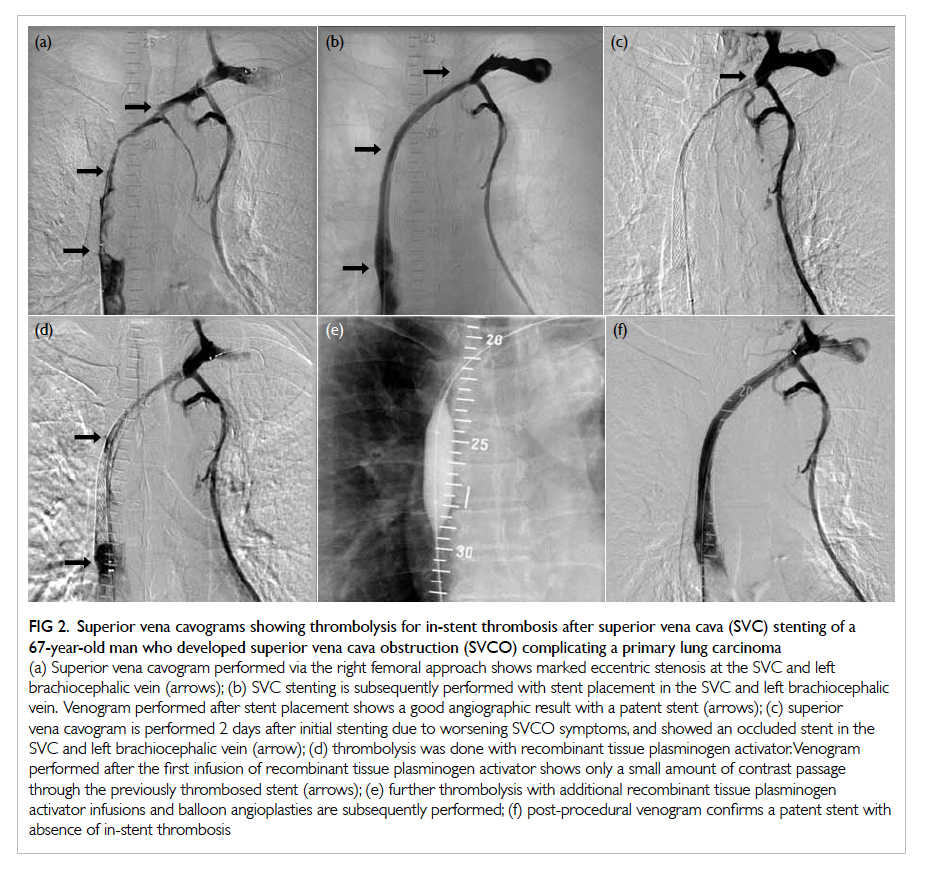
Figure 2. Superior vena cavograms showing thrombolysis for in-stent thrombosis after superior vena cava (SVC) stenting of a 67-year-old man who developed superior vena cava obstruction (SVCO) complicating a primary lung carcinoma
(a) Superior vena cavogram performed via the right femoral approach shows marked eccentric stenosis at the SVC and left brachiocephalic vein (arrows); (b) SVC stenting is subsequently performed with stent placement in the SVC and left brachiocephalic vein. Venogram performed after stent placement shows a good angiographic result with a patent stent (arrows); (c) superior vena cavogram is performed 2 days after initial stenting due to worsening SVCO symptoms, and showed an occluded stent in the SVC and left brachiocephalic vein (arrow); (d) thrombolysis was done with recombinant tissue plasminogen activator. Venogram performed after the first infusion of recombinant tissue plasminogen activator shows only a small amount of contrast passage through the previously thrombosed stent (arrows); (e) further thrombolysis with additional recombinant tissue plasminogen activator infusions and balloon angioplasties are subsequently performed; (f) post-procedural venogram confirms a patent stent with absence of in-stent thrombosis
Patients in the primary stenting group had a
statistically significant longer survival than patients
in the salvage stenting group (P<0.05). The median
survival was 64 (range, 5-1156) days for patients in
the primary stenting group, and 62 (range, 2-710)
days for patients in the salvage stenting group.
At the end of the data collection, one patient in
the primary stenting group was alive 1849 days after
stenting and two patients in the salvage stenting
group were alive 110 days and 226 days after stenting, respectively.
Two patients in the primary stenting group were lost
to follow-up.
Discussion
Symptoms of SVCO usually develop over a period of
2 weeks in approximately one third of patients, and
over a longer period in other patients. Oedema and
distended veins are the most common symptoms
and signs of SVCO of facial and arm oedema occurred
in 82% and 46% of patients, respectively, and neck
and chest vein distension occurred in 63% and 53% of
patients, respectively.1 Respiratory symptoms and
signs are common and include dyspnoea (54%),
cough (54%), hoarseness (17%), and stridor (4%).
Neurological symptoms and signs include syncope
(10%), headaches (9%), dizziness (6%), confusion
(4%), and visual symptoms (2%).1
Malignant conditions account for about 90% of
cases of SVCO in previous studies.12 Non–small-cell
lung cancer is the most common cause of malignant
SVCO and accounts for 50% of cases, followed by
small-cell lung cancer (22%), lymphoma (12%),
metastatic cancer (9%, of which two thirds are breast
cancer), germ-cell cancer (3%), thymoma (2%),
and mesothelioma (1%).1 Non-malignant causes
of SVCO have become more common in recent
years, reflecting the increasing use of intravascular
devices such as catheters and pacemakers.1 Ye et al13 have identified that most SVCOs of benign cause
are related to haemodialysis catheter placement
(70%). Other causes include hypercoagulability and
mediastinal fibrosis.
The femoral vein is the classic route for stent
insertion, and was used for most cases in the current
series. Some authors have also suggested jugular
vein, subclavian vein, or basilic vein access as possible
options.5 9 14 In cases of bilateral brachiocephalic
vein obstruction, some authors have proposed that
it is sufficient to relieve the obstruction by stent
placement in either the right or left brachiocephalic
vein, with collaterals allowing drainage from both
sides. It has been shown that this is as clinically
effective as bilateral stent placement, while offering
lower cost, easier placement, and lower rates of
complications and recurrence.5 7 8 9
Previous studies have shown 87% to 100%
effectiveness of primary stenting in relieving
SVCO. Recurrence of SVCO is seen in up to 18%
of patients.9 10 15 16 17 18 These figures are in keeping with
that shown in this study. After successful stent
placement, symptoms of SVCO usually resolve
within 48 to 72 hours. This is compared with
radiotherapy or chemotherapy which usually take
weeks to have an effect.1 12 The rapid improvement of patient’s haemodynamic and performance status
after primary stenting enables underlying aetiology-specific
therapy to be initiated at a full dose and in a
timely manner.2 In addition, primary SVC stenting
can also be performed immediately after diagnosis
in the absence of a histological diagnosis, which is
required for deciding the optimal treatment protocol
by conventional therapy with chemotherapy and/or
radiotherapy.
In patients with tumour recurrence or
progression despite conventional therapy, or
in patients who are not fit for chemotherapy or
radiotherapy due to poor performance status or
concomitant illness, salvage SVC stenting provides
good palliation of SVCO symptoms.19 Most studies
for salvage SVC stenting after conventional therapy
failure report effective relief of venous compression
after cancer recurrence, ranging from 81% to 100%,
which is similar to the findings in this study. Most
studies report a recurrence rate of up to 25% but
figures up to 33% to 41% have also been reported.2 20 21 22 23 24 25 26 27
A recent review article has studied complication
rates after SVC stenting.2 In a total of 884 stent
placements in 32 studies, the mortality was 2%,
which is similar to that in this study. A total of 41% of
the deaths were due to severe haemorrhage such as
pulmonary or cerebral haemorrhage, and 23% were
due to acute cardiac events, including arrhythmia,
myocardial infarction, and cardiac tamponade.
Other causes included respiratory failure (17%) and
pulmonary embolism (6%).2 Cardiac tamponade
following rupture of central veins, which was seen
in this series, is rare, but can be rapidly fatal.28 For
this reason, it has been suggested that facilities for
pericardial drainage should be available in the room
to allow emergent pericardiocentesis.12
Periprocedural and post-procedural
complications are low and were found in up to
19% of patients in previous studies.12 Overall, these
complications compare very favourably with those of
chemotherapy and radiotherapy.4 The most common
major complications are stent malposition or
migration, accounting for 47% of all complications,
followed by bleeding (21%), deep vein thrombosis
(10%), pulmonary oedema (8%), arrhythmia (5%),
infection (5%), and pulmonary embolism (3%).2
For stent-related complications, a series by
Lanciego et al7 reviewed 149 patients with Wallstent
placement for SVC syndrome, which demonstrated
a 10.7% rate of stent occlusion (complete, 8%; partial,
2.7%), 2.7% stent thrombosis, 2.7% stent shortening,
and 0.7% stent migration.
Although commonly given for patients
after SVC stent placement, the effectiveness of
anticoagulation has not been clearly proven. In
general, anticoagulation is recommended at least
for the first month after stent placement due to
the high thrombogenic effect of the stent before
neoendothelium covers the endovascular surface.7 A
range of 1 to 9 months of anticoagulation has been
proposed and no consensus is currently available.13
Patient survival is generally short and is related
to the usual status of locally advanced or metastatic
malignancy causing SVCO. As demonstrated in
this study, survival was shorter in patients receiving
salvage stenting after failure of conventional therapy
(mean, 3.7 months) compared with that of patients
receiving primary stenting before conventional
therapy (mean, 8.7 months). This is likely due to the
difference in underlying disease status between the
two groups of patients. In a previous report, overall
patient survival was approximately 6 months after
SVC stenting,7 which is similar to the overall mean
survival identified in this series (6.6 months).
There are a few limitations to this study. As a
retrospective study, there was a lack of standardised
selection criteria for the choice between primary SVC
stenting and conventional therapy by radiotherapy
and/or chemotherapy for patients presenting with
SVCO. There was also a lack of standardised grading
system of the degree of SVCO symptoms and
follow-up protocol. The decisions for angioplasty
before and after stent placement were made by the
operating radiologists during the procedure, and
the post-stenting anticoagulation regimen was also
decided individually by the senior physicians and
oncologists. The small sample size might have limited
the power of the study. There are also possibilities
of information bias during the review process. These
should serve as future references for performing
a prospective study with a standardised protocol
to evaluate the results of SVC stenting in different
groups of patients.
Conclusion
Stenting of SVC is a safe and effective means of
alleviating SVCO symptoms both in patients
undergoing primary stenting before conventional
therapy and in those undergoing salvage stenting
after failure of conventional therapy. The number
of stents required, success rates, procedure times,
symptom relief rates, complication rates, and re-procedure
rates showed no statistically significant
difference between these two groups of patients.
Declaration
All the authors have no potential conflict of interest to declare.
References
1. Wilson LD, Detterbeck FC, Yahalom J. Clinical practice.
Superior vena cava syndrome with malignant causes. N
Engl J Med 2007;356:1862-9. Crossref
2. Nguyen NP, Borok TL, Welsh J, Vinh-Hung V. Safety and
effectiveness of vascular endoprosthesis for malignant
superior vena cava syndrome. Thorax 2009;64:174-8. Crossref
3. Charnsangavej C, Carrasco CH, Wallace S, et al. Stenosis
of the vena cava: preliminary assessment of treatment with
expandable metallic stents. Radiology 1986;161:295-8. Crossref
4. Rowell NP, Gleeson FV. Steroids, radiotherapy,
chemotherapy and stents for superior vena caval
obstruction in carcinoma of the bronchus: a systematic
review. Clin Oncol (R Coll Radiol) 2002;14:338-51. Crossref
5. Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi
R. Superior vena caval stenting for SVC obstruction:
current status. Eur J Radiol 2009;71:343-9. Crossref
6. Fagedet D, Thony F, Timsit JF, et al. Endovascular treatment
of malignant superior vena cava syndrome: results and
predictive factors of clinical efficacy. Cardiovasc Intervent
Radiol 2013;36:140-9. Crossref
7. Lanciego C, Pangua C, Chacón JI, et al. Endovascular
stenting as the first step in the overall management
of malignant superior vena cava syndrome. AJR Am J Roentgenol 2009;193:549-58. Crossref
8. Dinkel HP, Mettke B, Schmid F, Baumgartner I, Triller J, Do
DD. Endovascular treatment of malignant superior vena
cava syndrome: is bilateral wallstent placement superior to
unilateral placement? J Endovasc Ther 2003;10:788-97. Crossref
9. Lanciego C, Chacón JL, Julián A, et al. Stenting as first
option for endovascular treatment of malignant superior
vena cava syndrome. AJR Am J Roentgenol 2001;177:585-93. Crossref
10. Nagata T, Makutani S, Uchida H, et al. Follow-up results
of 71 patients undergoing metallic stent placement for the
treatment of a malignant obstruction of the superior vena
cava. Cardiovasc Intervent Radiol 2007;30:959-67. Crossref
11. Nicholson AA, Ettles DF, Arnold A, Greenstone M, Dyet
JF. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol
1997;8:781-8. Crossref
12. Uberoi R. Quality assurance guidelines for superior vena
cava stenting in malignant disease. Cardiovasc Intervent
Radiol 2006;29:319-22. Crossref
13. Ye M, Shi YX, Huang XZ, Zhao YP, Zhang H, Zhang
JW. Endovascular recanalization of superior vena cava,
brachiocephalic, and subclavian venous occlusions caused
by nonmalignant lesions. Chin Med J 2012;125:1767-71.
14. Miller JH, McBride K, Little F, Price A. Malignant superior
vena cava obstruction: stent placement via the subclavian
route. Cardiovasc Intervent Radiol 2000;23:155-8. Crossref
15. Chatziioannou A, Alexopoulos T, Mourikis D, et al. Stent
therapy for malignant superior vena cava syndrome: should
be first line therapy or simple adjunct to radiotherapy. Eur
J Radiol 2003;47:247-50. Crossref
16. Bierdrager E, Lampmann LE, Lohle PN, et al. Endovascular
stenting in neoplastic superior vena cava syndrome prior
to chemotherapy or radiotherapy. Neth J Med 2005;63:20-3.
17. Gross CM, Krämer J, Waigand J, et al. Stent implantation
in patients with the superior vena cava syndrome. AJR Am
J Roentgenol 1997;169:429-32. Crossref
18. Chacón López-Muñiz JI, García García L, Lanciego Pérez C, et al. Treatment of superior and inferior vena cava
syndromes of malignant cause with Wallstent catheter
placed percutaneously. Am J Clin Oncol 1997;20:293-7. Crossref
19. Urruticoechea A, Mesia R, Dominguez J, et al. Treatment
of malignant superior vena cava syndrome by endovascular
stent insertion. Experience on 52 patients with lung cancer.
Lung Cancer 2004;43:209-14. Crossref
20. Kee ST, Kinoshita L, Razavi MK, Nyman UR, Semba CP,
Dake MD. Superior vena cava syndrome: treatment with
catheter-directed thrombolysis and endovascular stent
placement. Radiology 1998;206:187-93. Crossref
21. Marcy PY, Magné N, Bentolila F, Drouillard J, Bruneton JN,
Descamps B. Superior vena cava obstruction: is stenting
necessary? Support Care Cancer 2001;9:103-7. Crossref
22. Greillier L, Barlési F, Doddoli C, et al. Vascular stenting for
palliation of superior vena cava obstruction in non-small-cell
lung cancer patients: a future ‘standard’ procedure?
Respiration 2004;71:178-83. Crossref
23. García Mónaco R, Bertoni H, Pallota G, et al. Use of self-expanding vascular endoprosthesis in superior vena cava
syndrome. Eur J Cardiothorac Surg 2003;24:208-11. Crossref
24. Tanigawa N, Sawada S, Mishima K, et al. Clinical outcome
of stenting in superior vena cava syndrome associated
with malignant tumors. Comparison with conventional
treatment. Acta Radiol 1998;39:669-74. Crossref
25. Courtheoux P, Alkofer B, Al Refaï M, Gervais R, Le
Rochais JP, Icard P. Stent placement in superior vena cava
syndrome. Ann Thorac Surg 2003;75:158-61. Crossref
26. Crowe MT, Davies CH, Gaines PA. Percutaneous
management of superior vena cava occlusions. Cardiovasc
Intervent Radiol 1995;18:367-72. Crossref
27. Furui S, Sawada S, Kuramoto K, et al. Gianturco stent
placement in malignant caval obstruction: analysis of factors
for predicting the outcome. Radiology 1995;195:147-52. Crossref
28. Ploegmakers MJ, Rutten MJ. Fatal pericardial tamponade
after superior vena cava stenting. Cardiovasc Intervent
Radiol 2009;32:585-9. Crossref