DOI: 10.12809/hkmj144321
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Three cases of atypical pneumonia caused by Chlamydophila psittaci
Sandy Chau, MB, BS, FHKAM (Pathology)1;
Eugene YK Tso, MB, BS, FHKAM (Medicine)2;
WS Leung, MB, ChB, FHKAM (Medicine)2;
Kitty SC Fung, MB, ChB, FHKAM (Pathology)1
1 Department of Pathology, United Christian Hospital, Kwun Tong, Hong Kong
2 Department of Medicine and Geriatrics, United Christian Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr Sandy Chau (chauky@ha.org.hk)
Abstract
Psittacosis is a zoonotic disease caused by
Chlamydophila psittaci. The most common
presentation is atypical pneumonia. Three cases of
pneumonia of varying severity due to psittacosis are
described. All patients had a history of avian contact.
The diagnosis was confirmed by molecular detection
of Chlamydophila psittaci in respiratory specimens.
The cases showed good recovery with doxycycline
treatment. Increased awareness of psittacosis
can shorten diagnostic delay and improve patient
outcomes.
Introduction
Psittacosis is a zoonotic disease caused by
Chlamydophila psittaci, an obligate intracellular
pathogen belonging to the family Chlamydiaceae.
Since its first description in 1879, zoonotic
and enzoonotic outbreaks have been reported
worldwide.1 Transmission occurs through direct
contact or inhalation of aerosols from dried faeces,
feather dust, or respiratory secretions of infected
birds. Individuals with occupational or recreational
exposure to birds like bird fanciers and veterinarians
are at greatest risk of infection. Person-to-person
transmission is rare.2 The disease can range from
subclinical infection to fatal pneumonia. Here, we
report three cases of atypical pneumonia caused by
C psittaci in Hong Kong.
Case reports
Case 1
A 62-year-old retired male presented in March 2014
with fever, headache, myalgia, cough, and yellowish
sputum for 6 days. He had underlying diabetes
mellitus, hypertension, gout, and renal impairment.
He visited the local bird market frequently and had
purchased two parrots before onset of symptoms.
The parrots were well all along. On presentation,
he was alert and stable and his temperature was
38.4°C. The oxygen saturation was 96% on room
air. Chest examination revealed left lower zone
crepitations. Chest radiograph showed left lower
zone consolidation. He was suspected of psittacosis
and was treated with ceftriaxone and doxycycline.
His sputum was positive for C psittaci by polymerase
chain reaction (PCR). Nasopharyngeal swab and
sputum were also positive for influenza A virus
subtype H3 by PCR. Oseltamivir was commenced.
He was afebrile the next day and was discharged after
5 days of hospitalisation. Paired serum collected 12
days apart showed rising Chlamydia group titre
from 40 to 80 by complement fixation test (CFT).
The parrots could no longer be traced as the patient’s
son released them.
Case 2
A 55-year-old male construction site worker with
hypertension was admitted in February 2014 with a
1-week history of fever, headache, generalised bone
pain, and cough. He had travelled to Shanwei in
China and bought a live chicken from a wet market
2 weeks earlier. On examination, his vital signs were
stable and had a temperature of 40°C. The oxygen
saturation was 98% on room air. Chest examination
was normal. Chest radiograph showed right upper
zone opacities. He was treated as a case of community-acquired
pneumonia with amoxicillin-clavulanate,
doxycycline, and oseltamivir. Sputum culture
showed growth of commensals only. Nasopharyngeal
aspirate (NPA) was negative for influenza viruses,
Mycoplasma pneumoniae, and Chlamydophila
pneumoniae by PCR. Oseltamivir was stopped. His
fever subsided on day 3 and he was discharged after
4 days of hospitalisation. Complement fixation test
of paired sera taken 2 weeks apart showed a rise in
Chlamydia group titre from 20 to 80. Sputum was
retrieved and was positive for C psittaci by PCR.
Case 3
A 42-year-old female chef was hospitalised in
February 2014 with fever, cough, yellowish and
blood-stained sputum, and breathing difficulty for 1
week. She had no underlying illness. One week prior
to her admission, she had travelled to Zhaoqing in
China and bought live goose and chicken from a
wet market. On admission, she was in respiratory
distress and her temperature was 39.2°C. The oxygen
saturation was 90% on 100% supplemental oxygen
via non-rebreathing mask. Chest examination
yielded coarse crepitations over the right side. Chest
radiograph revealed right middle and lower zone
consolidation, and left patchy haziness (Fig). On
the same day, she was transferred to the intensive
care unit. She required mechanical ventilation and
extracorporeal membrane oxygenation (ECMO).
She was treated as a case of severe community-acquired
pneumonia with piperacillin-tazobactam,
doxycycline, and oseltamivir. Sputum culture
showed growth of commensals only. Sputum, NPA,
and tracheal aspirate were negative for influenza
viruses, M pneumoniae, and C pneumoniae by PCR.
Urine was negative for Legionella pneumophila and
pneumococcal antigens. She gradually improved
and was extubated 3 days later, and ECMO was
stopped on day 7. She was discharged on day 24 of
hospitalisation. Paired serum drawn 12 days apart
showed rising Chlamydia group titre from 80 to 640
by CFT. Polymerase chain reaction for C psittaci was
positive in her stored sputum.
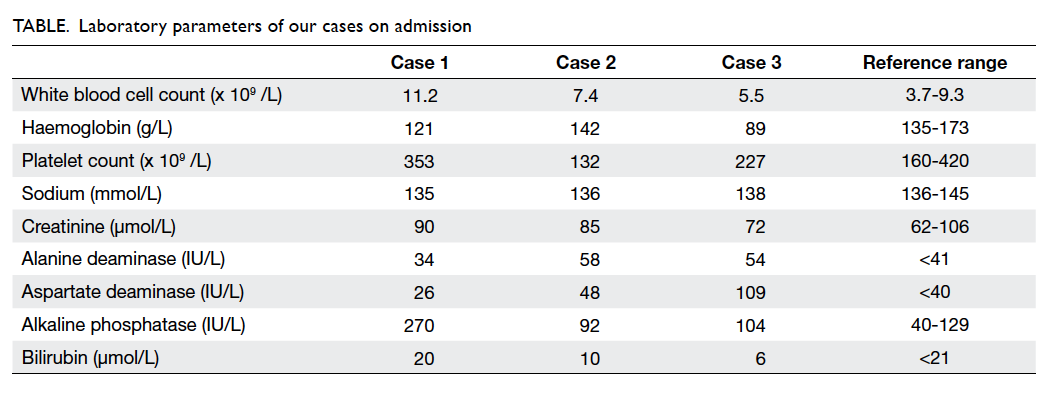
The laboratory profiles of the three cases on
admission are summarised in the Table.
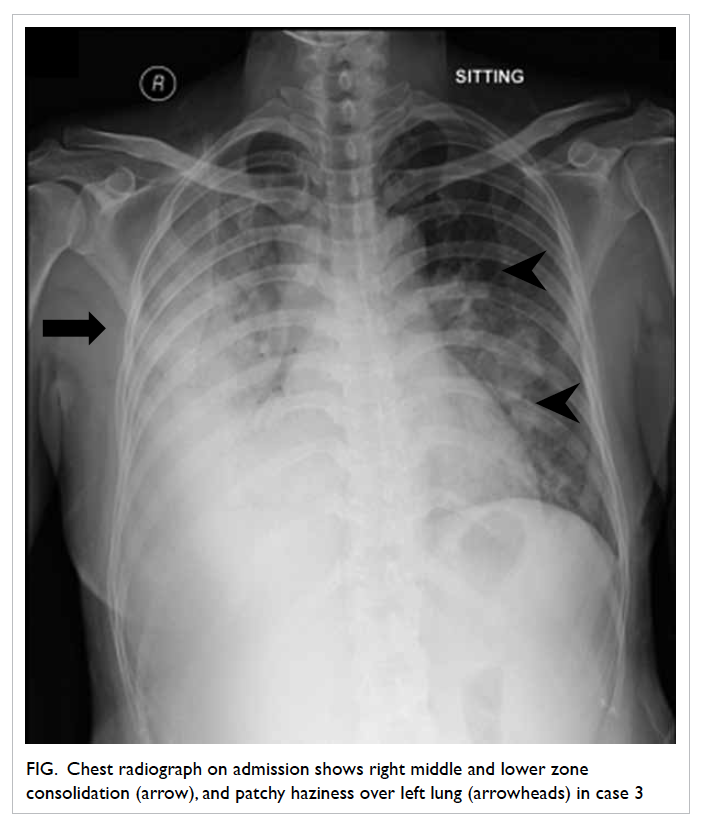
Figure. Chest radiograph on admission shows right middle and lower zone consolidation (arrow), and patchy haziness over left lung (arrowheads) in case 3
Discussion
Psittacosis is an uncommon disease in Hong Kong.
Since 2008, this has been made as a statutory
notifiable disease. There were 11 confirmed cases
and six probable cases reported until 2013.3 In 2012,
there was an outbreak involving six staff working
at the New Territories North Animal Management
Centre in Sheung Shui.4
Chlamydophila psittaci is classified into
nine genotypes (A to F, E/B, M56, WC) based on
outer membrane protein A gene sequences, with
differential host preference and virulence among the
genotypes.1 It has been described in at least 460 bird
species from 30 orders.5 Birds can shed the organisms
when apparently healthy or overtly ill. Patient 1 had
been exposed to asymptomatic parrots, belonging
to the classic culprits, the psittacine birds (parrots,
parakeets, budgerigars, cockatiels). Other domestic
species—for examples, turkey, pigeon, goose, duck,
chicken—can be affected and are often overlooked
as potential reservoirs of infection.6 In patients
2 and 3, psittacosis was not suspected initially
although the patients had been exposed to poultry
in China. Chlamydophila psittaci is an emerging
pathogen among chicken.7 In China, the prevalence
of infection among market-sold chickens, ducks,
and pigeons has been reported to be 13.32%, 38.92%
and 31.09%, respectively.8 The substantial zoonotic
transmission risk from domestic species should also
be recognised.
Psittacosis is a systemic illness that affects
several organ systems, and atypical pneumonia as in
our cases is the most common manifestation. Patients
typically present with influenza-like symptoms,
which include high fever, headache, myalgia, and
dry cough. Patient 3 complained of blood-stained
sputum, which may occur occasionally.1 9 The headaches can be so severe as to suggest meningitis
on presentation. Diarrhoea is common and can be
the chief presenting complaint. Relative bradycardia,
Horder’s spots, and splenomegaly are characteristic
physical signs. There may be disparity between the
auscultatory findings and radiographic changes of
pneumonia. Segmental consolidation in lower lobe
is the most common radiographic abnormality
although normal chest radiograph has been reported
in over 20% of cases. Hilar lymphadenopathy and
pleural effusion are rare.10 The white cell count
is usually normal or slightly raised, with mildly
abnormal liver function. Our case presentations
were consistent with psittacosis. However, it is
indistinguishable clinically from other causes of
atypical pneumonia like C pneumoniae. The severity
ranged from mild-to-severe pneumonia requiring
intensive care management and ECMO in our cases.
Patient 1 had mild illness although co-infected
with influenza A. This diversity of presentation is
in agreement with other case series.9 The mortality
rate can be approximately 15% to 20% without
appropriate treatment but if properly treated, it is
rarely fatal.11 Extrapulmonary complications such as
endocarditis, myocarditis, renal disease, hepatitis,
keratoconjunctivitis, arthritis, and encephalitis have
also been described.
Chlamydophila psittaci is not covered during
routine bacterial or viral workup. Definitive
diagnosis can only be established by culture,
serology, or PCR specifically targeting on C psittaci.
Culture is time-consuming and requires level-3
biosafety facilities. Common serological assays
include CFT, enzyme-linked immunosorbent assay,
and microimmunofluorescence (MIF) test. The
assays are neither sensitive nor specific but MIF test
is regarded as more specific.12 However, there are
still considerable cross-reactions between different
species of the Chlamydiaceae family.1 Besides, a
convalescent serum obtained at least 2 weeks apart
is required to demonstrate the 4-fold rise in titre.
In patient 1, the elevation in CFT titre was not
significant and therefore diagnosis may be missed if
relying on serology alone. Early use of doxycycline
in this patient might have blunted the antibody
response.13 In patients 2 and 3, serology results were
available only retrospectively; PCR for C psittaci was
performed subsequently to confirm the diagnosis.
In our cases, a nested PCR based on 16S rRNA
gene was used. The first-step PCR is genus-specific,
followed by the second-step PCR that can detect C
psittaci specifically.14 This method was demonstrated
to be sensitive and specific for detection of C
psittaci. When encountering respiratory illness with
suspicion of psittacosis, PCR testing on respiratory
specimens can offer a rapid and specific diagnosis.
Tetracyclines, in particular doxycycline, are
considered to be the treatment of choice. In patients
presenting with community-acquired pneumonia,
addition of doxycycline to a beta-lactam–based
empirical regimen can provide coverage for
both psittacosis and other atypical pathogens.
Defervescence usually takes place within 48 hours
of treatment. Our patients showed significant
improvement by day 3 of doxycycline treatment. The
commonly recommended duration of treatment is at
least 10 to 21 days to prevent relapse.9 Macrolides
can be used as alternative therapy, but may be
less efficacious in severe cases and gestational
psittacosis.6 Although quinolones have in-vitro
activity against C psittaci, their clinical effectiveness
remains to be determined.6
Conclusion
Psittacosis often goes unrecognised because of the
lack of distinctive symptoms and clinical suspicion.
In patients with atypical pneumonia, a history of
exposure to birds gives a very valuable diagnostic
clue. Early diagnosis with PCR testing and timely
initiation of appropriate antibiotics can reduce
patient morbidity and mortality.
Acknowledgement
We thank the Public Health Laboratory Services
Branch of the Centre for Health Protection for
providing the laboratory testing of psittacosis.
References
1. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila
psittaci infections from a clinical perspective. Clin
Microbiol Infect 2009;15:11-7. Crossref
2. Hughes C, Maharg P, Rosario P, et al. Possible nosocomial
transmission of psittacosis. Infect Control Hosp Epidemiol
1997;18:165-8. Crossref
3. Statistics on communicable diseases. Available from:
http://www.chp.gov.hk/en/notifiable1/10/26/43.html. Accessed 25 Feb 2015.
4. Psittacosis outbreak in an animal management centre,
2012. Communicable Diseases Watch 2013;10:9-10.
Available from: http://www.chp.gov.hk/files/pdf/cdw_compendium_2013.pdf. Accessed 25 Feb 2015.
5. Kaleta EF, Taday EM. Avian host range of Chlamydophila
spp. based on isolation, antigen detection and serology.
Avian Pathol 2003;32:435-61. Crossref
6. Stewardson AJ, Grayson ML. Psittacosis. Infect Dis Clin
North Am 2010;24:7-25. Crossref
7. Lagae S, Kalmar I, Laroucau K, Vorimore F, Vanrompay D.
Emerging Chlamydia psittaci infections in chickens and
examination of transmission to humans. J Med Microbiol
2014;63:399-407. Crossref
8. Cong W, Huang SY, Zhang XY, et al. Seroprevalence
of Chlamydia psittaci infection in market-sold adult
chickens, ducks and pigeons in north-western China. J
Med Microbiol 2013;62:1211-4. Crossref
9. Yung AP, Grayson ML. Psittacosis—a review of 135 cases.
Med J Aust 1988;148:228-33.
10. Coutts II, Mackenzie S, White RJ. Clinical and radiographic
features of psittacosis infection. Thorax 1985;40:530-2. Crossref
11. Smith KA, Campbell CT, Murphy J, Stobierski MG,
Tengelsen LA. Compendium of measures to control
Chlamydophila psittaci (formerly Chlamydia psittaci)
infection among humans (psittacosis) and pet birds (avian
chlamydiosis), 2010 National Association of State Public
Health Veterinarians (NASPHV). J Exotic Pet
Med 2011;20:32-45. Crossref
12. Petrovay F, Balla E. Two fatal cases of psittacosis caused by
Chlamydophila psittaci. J Med Microbiol 2008;57:1296-8. Crossref
13. Heddema ER, van Hannen EJ, Duim B, et al. An outbreak
of psittacosis due to Chlamydophila psittaci genotype
A in a veterinary teaching hospital. J Med Microbiol
2006;55:1571-5. Crossref
14. Messmer TO, Skelton SK, Moroney JF, Daugharty H, Fields
BS. Application of a nested, multiplex PCR to psittacosis
outbreaks. J Clin Microbiol 1997;35:2043-6.